It’s time to choose
Tailored and Flexible Dosing
APLENZIN—the ONLY bupropion HBr with no generic substitute
Write "DAW1" on a prescription to ensure it is dispensed as written.
APLENZIN is a convenient once-daily, single tablet with no generic substitute1,2
APLENZIN has been demonstrated to be bioequivalent to bupropion HCl extended-release.1
3 dosage strengths with simple dose titration and transition from another bupropion1
One pill size and less pill burden at any dosage strength, with no food restrictions
Dosing recommendations for APLENZIN
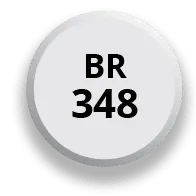
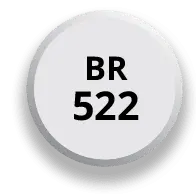
After 4 days of 174 mg, the dose may be increased to the target dose of 348 mg once daily in the morning
After 4 days of 174 mg, the dose may be increased to the target dose of 348 mg once daily in the morning
Initiate dosing in the fall, prior to onset of SAD symptoms
After 7 days of 174 mg, the dose may be increased to the target dose of 348 mg once daily in the morning*
Continue treatment throughout winter and taper/discontinue in early spring†
*Doses above 300 mg of bupropion HCI extended-release (equivalent to APLENZIN 348 mg) were not assessed in the SAD trials.1
†For patients treated with 348 mg per day, decrease the dose to 174 mg once daily before discontinuing APLENZIN.1
References:
1. APLENZIN (bupropion hydrobromide) extended-release tablets Prescribing Information. Bausch Health Companies Inc.
2. Approved Drug Products With Therapeutic Equivalence Evaluations. 43rd ed. U.S. Department of Health and Human Services, Food and Drug Administration. 2023.
3. Straka RJ, Keohane DJ, Liu LZ. Potential clinical and economic impact of switching branded medications to generics. Am J Ther. 2017;24(3):e278-e289.
APLENZIN® (bupropion hydrobromide extended-release tablets) is indicated for the treatment of major depressive disorder (MDD), and for the prevention of seasonal major depressive episodes in patients with a diagnosis of seasonal affective disorder (SAD). Periodically reevaluate long-term usefulness for the individual patient.
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
SUICIDALITY AND ANTIDEPRESSANT DRUGS:
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term trials. These trials did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in subjects aged 65 and older.
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
APLENZIN is contraindicated in:
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
SUICIDALITY AND ANTIDEPRESSANT DRUGS:
APLENZIN® (bupropion hydrobromide extended-release tablets) is indicated for the treatment of major depressive disorder (MDD), and for the prevention of seasonal major depressive episodes in patients with a diagnosis of seasonal affective disorder (SAD). Periodically reevaluate long-term usefulness for the individual patient.
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
SUICIDALITY AND ANTIDEPRESSANT DRUGS:
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term trials. These trials did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in subjects aged 65 and older.
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
APLENZIN is contraindicated in:
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
You are now leaving the APLENZIN website and are going to a website that Bausch Health does not operate and to which Bausch Health’s Privacy Policy does not apply. Bausch Health is not responsible for the content, format, maintenance, or policies of the website you are about to visit and does not endorse or monitor any content on such website.
YOU ARE NOW ENTERING THE APLENZIN HEALTHCARE PROFESSIONAL SITE.
The information contained in this site is technical in nature and was designed specifically for healthcare professionals.
To proceed, please choose one of the following: